prevent cognitive decline
The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) is the first trial in the world to demonstrate that multidomain lifestyle interventions can improve our brain health and prevent cognitive decline.
With professor Miia Kivipelto as Principal Investigator, the study is a collaboration between Karolinska Institutet, the Finnish Institute for Health and Welfare, the University of Eastern Finland, the University of Turku, the University of Helsinki, the University of Oulu, and Umeå University.
The results of the FINGER study were first published in the Lancet medical journal in 2015 (Ngandu, Kivipelto, et al.).
Randomized controlled trial
FINGER was conducted as a double-blind, randomized controlled trial with 1,260 participants, aged 60-77. All participants had an increased risk of dementia, based on certain identified risk factors, but they had no obvious memory problem. They were randomly divided into two arms, one intervention group and one control group.
The intervention group went through a two-year program of simultaneous multidomain lifestyle interventions in the areas of diet, physical activity, cognitive training, social activity, and monitoring of cardiovascular risk factors. The control group received regular health advice.

Multimodal lifestyle interventions
In the intervention program, participants were educated and supported in eating a healthy balanced diet based on the Nordic Nutrition Recommendations.
The physical training interventions included regular cardio, strength, and balance training, both in groups and individually.
Cognitive stimulation and training were provided as online programs covering several areas, including memory and processing speed.
The participants’ cardiovascular health status, including blood pressure, cholesterol, blood glucose, and obesity, was regularly monitored. The social activity portion of the interventions came natural as part of group sessions and meetings.
Extended follow-ups are being conducted up to 11 years.
Results and findings
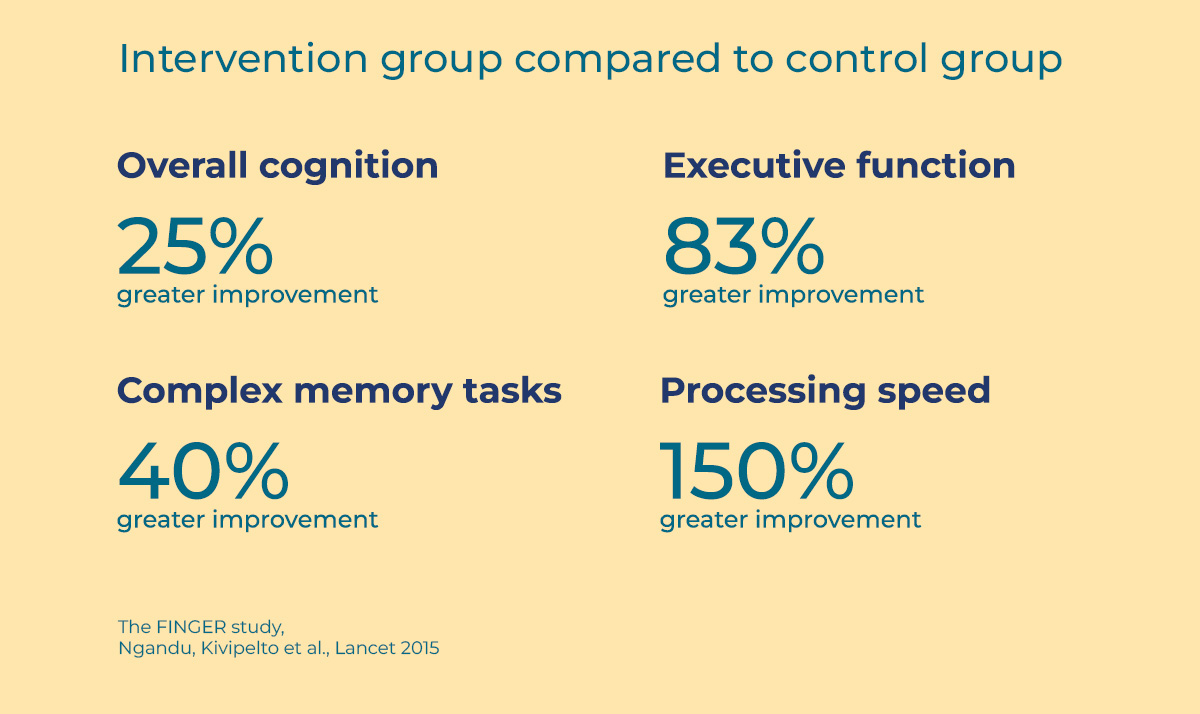
Cognitive performance improved among participants in both groups, but the total average improvement of the intervention group was 25% greater than the improvement of the control group.
To highlight some specifically noteworthy numbers, there was an 83% greater improvement in executive function, 150% greater improvement of psychomotor speed, and 40% greater improvement of complex memory tasks in the intervention group compared to the control group (Ngandu, Kivipelto et al, 2015). Carriers of the ApoE 4 gene allele had clear benefits of the interventions (Solomon et al., 2018).
In the control group, there was a 30% greater risk of developing cognitive impairment after two years, compared to the intervention group.
In addition to directly improving cognitive health, the FINGER intervention program had positive effects on the participants’ mobility and their ability to cope well with day-to-day activities (Kulmala et al., 2019). The intervention group participants reported better health-related quality of life (Strandberg et al., 2017), and the risk of multimorbidity was reduced by 60% compared to the control group (Marengoni et al., 2018). Lifestyle changes were more significant in the intervention group, and active participation was associated with higher benefits of the intervention (Ngandu et al., 2022).
Over a longer post-intervention follow-up, the FINGER intervention also resulted in less cerebrovascular events (Lehtisalo et al., 2022).
Modeling studies support that programs like FINGER have the potential to be cost-effective in preventing dementia and that the societal benefits can be substantial (Wimo et al., 2022).
Why lifestyle interventions work
So what are the mechanisms behind the positive effects of the FINGER model? The FINGER research teams are still working to understand the multifactorial details of those processes, but one defined component is the improved blood circulation in the brain, and the reduced risk for inflammation and oxidative stress, thanks to improved cardiovascular health.
It is also believed that the FINGER interventions have direct effect on the brain’s resilience, supporting the strengthening of the cognitive reserve and the compensation for nerve cell changes and damage.
Coordinated by THL, Finland
The Finnish Institute for Health and Welfare, THL, is the coordinating site for FINGER.
THL manages the contacts with the FINGER participants and holds the trial register.
From FINGER
to World-Wide FINGERS
The FINGER research is tested and further adapted and optimized worldwide.
In 2017, Professor Miia Kivipelto launched the World-Wide FINGERS network, which has expanded to now include research teams from over 60 countries. So far, 13 trials have been completed around the world, 15 are currently ongoing (June 2023), and several are planned.



